Sex determination and formation of gonads
The question of whether a person develops anatomically as a male or female mostly depends on whether testes or ovaries form during embryonic life. In males, a key gene from the Y chromosome triggers a series of events that cause embryonic gonad cells to assemble into testes, whereas in females, different genetic and cellular events occur to make ovaries develop. Once testes or ovaries form, they make hormones that control other anatomical differences between male and female. So, sex development is a complex, stepwise process, and things don't always go according to plan.
Although the dice are cast at the moment of conception regarding what combination of sex chromosomes are inherited from the parents, it takes some time before an embryo’s biological sex begins to unfold.
Male and female human embryos look identical for the first six weeks of their development. After that, internal embryonic structures called gonadal ridges start to mature, usually forming either a pair of testes (the plural of testis: one testis, two testes) or a pair of ovaries. This is the first sign that the embryo is starting to follow either the male or female pathway of development, respectively.
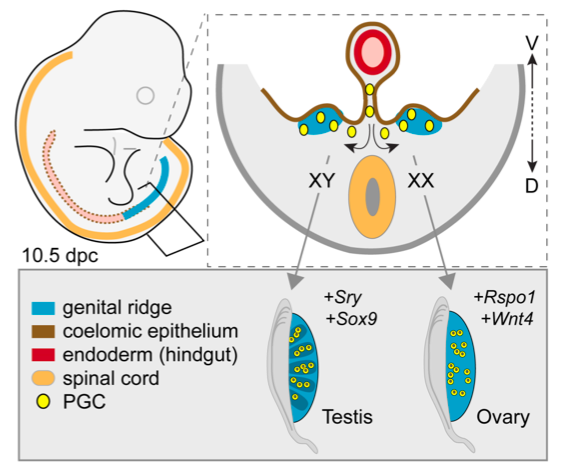 Formation of the testes or ovaries from embryonic gonadal ridges.
Formation of the testes or ovaries from embryonic gonadal ridges.
Top left: Illustration of a mouse embryo about 10 days after fertilization, which is similar in shape and size to a human embryo at an analogous stage (about 6 weeks gestation). The position of the gonadal ridges (sometimes called genital ridges) is shown in blue. Top right: Diagram of a cross-section of the embryo, showing the gonadal ridges in blue. Bottom right: Each gonadal ridge can develop into a testis or an ovary, depending on the genetic signals received. In XX embryos, ovaries usually form, but in XY embryos, testes usually form.
Like most events that occur during embryonic life, the choice of which pathway to follow — the process of sex determination — is controlled by genes that need to act in the right place, and at the right time. In the case of sex determination, certain genes must become active in a group of cells inside the gonadal ridges between the 6th and 8th weeks of gestation. In XX embryos, specific genes in these cells start to activate the chain of events that typically lead to the formation of ovaries. It’s interesting to note that XY embryos have all the same “ovary” genes too, but those genes are normally not activated. What happens instead in XY embryos is a chain of events that depends on the one thing that XX embryos don’t have — the Y chromosome.
Male sex determination
Although there are dozens of genes present on the Y chromosome, only one is needed to trigger male sex determination. That gene is called SRY.
The function of SRY is to activate another gene called SOX9, which in turn has many functions. SOX9 switches on a large set of genes required for making testes (see diagram below). At least a dozen genes are currently known to be critical for testis development, and that is likely to be just the tip of the iceberg, with many more “testis” genes still to be discovered. The other function of SOX9 is to suppress the genes responsible for making ovaries; because those genes are present in both sexes, they need to be actively silenced in the case of male embryos.
To be even more specific, SRY and SOX9 provide the instructions that direct only one of several different types of cell in the gonadal ridges how to differentiate. Those cells become Sertoli cells, the specialised cells of the testes that form tubules which protect and nourish developing sperm and, eventually, provide a route for the sperm cells to move out of the testes. Once Sertoli cells form, they produce chemical signals that instruct the other immature cells in the genital ridges to develop into specialized cell types important for testis function. These cell types include hormone-producing Leydig cells (see below), and sperm cells. Sertoli cells also generate other chemical signals that pass out of the developing testes to promote the formation of some internal sex organs characteristic of males (such as the ducts that carry the sperm).
The Leydig cells, by making testosterone, help to guide the maturation of the male internal reproductive duct system, and influence the external genitals to develop as a penis and a scrotum. Production of testosterone first occurs automatically in the Leydig cells, but as the testis matures, this process needs to be stimulated by hormones from other glands, such as luteinizing hormone (LH) produced in the pituitary gland.
In summary, between 6 and 8 weeks of human embryo development is a period of intense genetic and cellular activity during which testes are formed from the gonadal ridges in XY embryos. This starts the process of anatomical development of maleness.
Female sex determination
During the same period, in XX embryos, the gonadal ridges are usually busy developing into ovaries. A set of “pro-ovary” genes (such as Wnt4, Rspo1 and FoxL2; see diagram below) becomes active in a subset of cells that develops into granulosa cells. Just as Sertoli cells surround and protect sperm cells in the testis, it is the granulosa cells in the ovary that nurse the developing oocytes. Other genes cause some immature cells of the gonadal ridges to become hormone-producing Theca cells later required for female-specific development and reproductive functions. Currently, much less is known about ovary development compared to testis development.
Sex development is a complex, stepwise process
It should be clear from the above discussion that the development of typical male or female characteristics relies on a series of stepwise events that starts with the activity of a handful of genes in a few cells of the genital ridges, reaches an important milestone usually marked by the formation of testes or ovaries (part of the process of primary sex determination), and involves complex signals that favour the formation of sex-appropriate internal ducts and external genitalia (part of the process of secondary sex determination). This series of events is depicted in the following diagram.
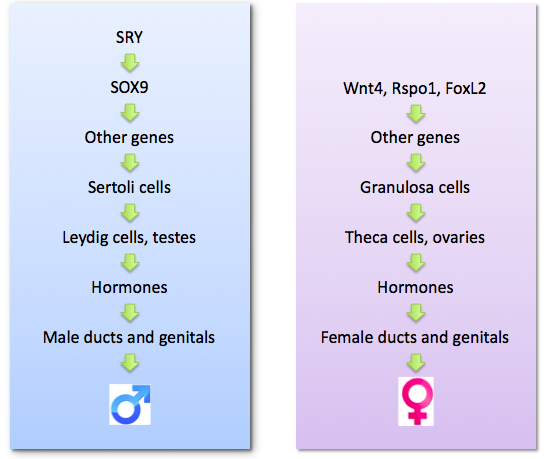
As mentioned above, some genes involved in promoting male sex determination and gonadal differentiation have an additional role in suppressing the female-specific development, which would otherwise tend to proceed. Research involving mice, whose sex development is similar to that of humans, has shown not only that “maleness” genes actively oppose “femaleness” genes, but also that femaleness genes play a lifelong role in suppressing maleness genes. These mechanisms work to maximise the probability that all of the cells in an embryonic testis will develop into cell types appropriate for testes as opposed to ovaries, and that all the cells in embryonic ovaries will develop into appropriate ovarian cell types.
In other words, there are built-in mechanisms to maximise the chance that the biological sex of the gonads, and hence sex of the embryo, ends up as fully male or female, rather than something in-between or unclear.
Nonetheless, occasionally things don’t go according to plan, and a mixture of testicular and ovarian cell types comes to occupy the same gonad. When that happens, the resulting gonad is referred to as an ovotestis. When ovotestes form, the amount or balance of hormones produced may not allow the formation of typical male or female internal ducts or external genitals. In addition, ovotestes may not be able to produce gametes (sperm or oocytes).
It should also be noted that because each embryo usually has two gonads, the detailed, stepwise process of testis or ovary formation needs to unfold twice, on the right and on the left, independently but at the same time. Occasionally one gonad may take a different developmental path to the other gonad. This most often takes the form of an ovotestis on one side and a testis or ovary on the other—a situation called unilateral ovotestis.
-----------------------------------------------
Last updated: 4 August 2021 PK
Edit history: Author P. Koopman 9/2012; revised PK 5/2013, 10/2013, 7/2015